Abstract
In classical Hodgkin lymphoma (CHL) the tumor microenvironment (TME) is enriched in T cells that modulate antitumor immunity. PD1 blockade partially restores anti-tumoral T cell function, to induce impressive responses in a proportion of patients with relapsed/refractory CHL (Chen et al JCO 2017). Further characterisation of T cell immune evasion mechanisms in CHL will permit the rational development of enhanced immunotherapeutic strategies. Lymphocyte-activation gene 3 (LAG3) is a cell surface molecule known to be expressed on a subset of immune effector T cells and intratumoral regulatory T cells (Tregs) in solid-organ tumors, with combination PD1/LAG3 mAb blockade showing early promise (Ascierto et al 2017 JCO abst 9520). In contrast, data in haematological malignancies is limited, although it is known that LAG3+ T cells suppress anti-tumoral immunity in CHL and B-CLL (Gandhi et al Blood 2006; Shapiro et al Haematologica 2017). Interestingly, in B-CLL LAG3 is found on both T cells and malignant B cells. Whether Hodgkin Reed-Sternberg (HRS) cells express LAG3 is unknown.
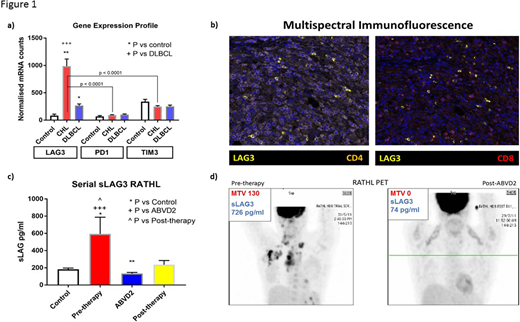
To characterise in detail intratumoral and circulating LAG3 in CHL we used a conventional discovery / validation approach. The local institutional discovery cohort was drawn from Princess Alexandra Hospital in Brisbane (Australia), and validated in samples from the prospective randomised phase III international "RATHL" trial (Johnson et al NEJM 2016). Firstly, LAG3 gene expression (GEP) was digitally quantified by NanoString in FFPE tissues in the discovery cohort and compared to normal control nodes and DLBCL tissues. Normalised LAG3 mRNA counts were 5-10-fold higher in CHL than in controls (P < 0.001) and 3-5-fold higher than in DLBCL (P < 0.001), whereas PD1 and TIM3 mRNA counts did not differ. In CHL samples LAG3 mRNA counts were markedly increased compared to PD-1 axis molecules and TIM3 (P < 0.001) (Figure 1a). Higher levels of LAG3 mRNA counts were correlated with infiltration by T cells (CD4 r = 0.55; P < 0.00001; CD8 r = 0.51, P < 0.0001), and macrophages (CD68 r = 0.45; P = 0.002). Findings were replicated in the RATHL cohort.
Next, intratumoral LAG3 cellular distribution was established. Flow cytometry was used to quantify LAG3 in T cell subsets and CD30+CD3- HRS cells in 6 de-aggregated freshly frozen CHL nodes (TILs). LAG3 was evenly distributed between CD8+ T cells, CD127LOCD25HI natural-Tregs (nTregs) and CD127LOCD25LO induced-Tregs (iTregs), but with minimal expression on CD4 non-Tregs, with the latter constituting the majority of intratumoral T cells. LAG3+ T cells typically co-expressed PD1 and/or TIM3. LAG3 was expressed on CD30+CD3+ cells but not on CD30+CD3- cells, consistent with LAG3 expression on activated T cells. Multispectral immunofluorescence (mIF) image analysis confirmed these findings in histological tumor samples (Figure 1b). Also, there was negligible expression of LAG3 on HRS-lines.
Finally, the potential role of soluble LAG3 (sLAG3) as a rapid-turnaround circulating biomarker applicable to the routine diagnostic laboratory, was assessed in serum samples using the MSD R-PLEX assay. In the discovery cohort sLAG3 was 3-4-fold increased at pre-therapy compared to controls and 3-6M post-therapy serum (P = 0.001). Results from pre-therapy RATHL serum samples were similar (P < 0.05). Notably in RATHL samples at interim restaging after 2 ABVD cycles sLAG3 had reduced by ~5-fold compared to pre-therapy (P < 0.0001) in patients with PET/CT responsive disease (Figures 1 c + d). Twelve months post therapy sLAG remained significantly lower than pre-therapy (P < 0.05) and was equivalent to control samples. Pre-therapy serum sLAG3 demonstrated a modest correlation with tissue LAG3 mRNA counts (r = 0.45; P = 0.02).
In conclusion in CHL, LAG3 mRNA expression was markedly increased relative to control and DLBCL tissues. Within CHL tissues LAG3 mRNA was markedly increased compared to other immune checkpoint molecules. Interrogation of the TME using flow cytometry of TILs and mIF demonstrated LAG3 is evenly distributed between CD8+, nTregs and iTRreg. In tumor samples we did not find evidence of LAG3 expression on HRS cells. To our knowledge this is the first study to demonstrate sLAG3 as a cell free circulating disease response biomarker in CHL. Taken together these findings provide a convincing rationale for further exploration of single and/or combined checkpoint blockade incorporating LAG3 inhibition to treat CHL.
Abro:Bristol-Myers Squibb: Speakers Bureau; Janssen: Other: education support congress attendance; Celgene: Other: education support congress attendance; Novartis: Consultancy; Amgen: Other: education support congress attendance. Keane:BMS: Research Funding; Roche: Other: Education Support, Speakers Bureau; Celgene: Consultancy, Research Funding; Takeda: Other: Educational Meeting; Merck: Consultancy. Birch:Medadvance: Equity Ownership. Tobin:Amgen: Other: Educational Travel; Celgene: Research Funding. Johnson:Kite: Consultancy; Celgene: Honoraria; Eisai: Research Funding; Incyte: Consultancy; Takeda: Honoraria, Travel, accommodations, expenses; Janssen: Consultancy, Research Funding; Genmab: Consultancy; Novartis: Honoraria; Zenyaku Kogyo: Other: Travel, accommodations, expenses; Bristol-Myers Squibb: Honoraria; Boeringher Ingelheim: Consultancy; Epizyme: Consultancy, Honoraria, Research Funding. Trotman:F. Hoffman-La Roche: Other: Travel to meeting, Unremunerated member of Ad Board, Research Funding; PCYC: Research Funding; Janssen: Other: Unremunerated member of Ad Board, Research Funding; Takeda: Other: Unremunerated member of Ad Board; Celgene: Other: Unremunerated member of Ad Board, Research Funding; Beigene: Research Funding. Bird:Amgen, Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Gill:Janssen: Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Speakers Bureau. Gandhi:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Takeda: Honoraria; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal